Covid Vaccine Pregnancy Research Studies - Pfizer Biontech To Trial Covid 19 Vaccine In Pregnant Women And Children
Researchers will measure the development and durability of antibodies against Covid-19 in people vaccinated during pregnancy or in the first two months after delivery. A new observational study has begun to evaluate the immune responses generated by COVID-19 vaccines administered to pregnant or postpartum people.
The Incidence Severity And Management Of Covid 19 In Critically Ill Pregnant Individuals Ontario Covid 19 Science Advisory Table
The exposed group Cohort 1 includes pregnant women who received at least one dose of a COVID-19 vaccine from 30 days prior to the first day of the LMP to end of pregnancy.
Covid vaccine pregnancy research studies. Studies show that protection against SARS-CoV-2 begins to decrease over time after initial vaccine doses. This study aimed to evaluate the immunogenicity and reactogenicity of coronavirus disease 2019 messenger RNA vaccination in pregnant and lactating women compared with. Researchers also report similar pregnancy outcomes including for preterm birth.
They find that pregnant individuals didnt report having any more severe reactions to the COVID-19 vaccines compared with women who were not pregnant except for nausea and vomiting which were reported slightly more frequently among vaccinated pregnant individuals after the second dose of the vaccine. There is no evidence of adverse maternal or fetal effects from vaccinating pregnant individuals with COVID-19 vaccine and a growing body of data demonstrate the safety of such use Ciapponi 2021 Wainstock 2021 Kachikis 2021 Magnus 2021. But the data that speak most clearly to the question of whether the COVID-19 vaccines harm fertility come from the clinical trials themselves 46.
Spontaneous abortions did not have an increased odds of exposure to a COVID-19 vaccination in the prior 28 days compared with ongoing pregnancies adjusted odds ratio 102. Early data suggest receiving an mRNA COVID-19 vaccine during pregnancy reduces the risk for infection. New study into COVID-19 vaccine dose interval for pregnant women.
1 nonpregnant controls and 2 natural coronavirus disease 2019 infection in pregnancy. Therefore individuals who are or will be pregnant should receive the COVID-19 vaccine. Researchers will measure the development and durability of antibodies against SARS-CoV-2 the virus that causes COVID-19 in people vaccinated during pregnancy or the first two postpartum months.
This study provides evidence to support the safety and efficacy of COVID-19 vaccination in pregnancy with protection to the neonate against infection outlining clear vaccine benefits for both maternal and child health. In June the US National Institutes of Health NIH started MOMI-VAX a new clinical study to assess immune responses induced by Covid-19 vaccines in pregnant or postpartum individuals. Researchers also will assess.
Overall the data on COVID-19 vaccines in pregnant people are still limited but growing. Additional vaccine doses booster vaccinations provide longer-lasting protection against COVID-19The FDA has authorized booster vaccinations of all three COVID-19 vaccines available in the United States. A new CDC analysis external icon of current data from the v-safe pregnancy registry assessed vaccination early in pregnancy and did not find an increased risk of miscarriage among nearly 2500 pregnant women who received an mRNA COVID-19 vaccine before 20 weeks of pregnancy.
100 Percent Fed Up After re-analyzing a study performed by Centers for Disease Control and Prevention CDC researchers a peer-reviewed study has called for the immediate withdrawal of mRNA COVID vaccines for pregnant women those breastfeeding those of childbearing age and children after their shocking study reveals stunning results of pregnant mRNA vaccinated women. Vaccine safety monitoring systems. A COVID-19 vaccine was received within 28 days prior to an index date among 80 of ongoing pregnancy periods vs 86 of spontaneous abortions.
Pregnant people were shut out of Covid vaccine trials with disastrous results Only about 34 percent of pregnant adults are fully vaccinated and more than 200 have died of the virus according. Extensive real-world data shows vaccines are safe and highly effective for pregnant women. The target study population consists of pregnant women who are 18 years of age or older.
Our study found no evidence of an increased risk for early pregnancy loss after Covid-19 vaccination and adds to the findings from other reports supporting Covid. Clinical trials have shown that COVID-19 vaccines are remarkably effective in protecting those age 12 and up against infection by the coronavirus SARS-CoV-2. The v-safe after vaccination health checker surveillance.
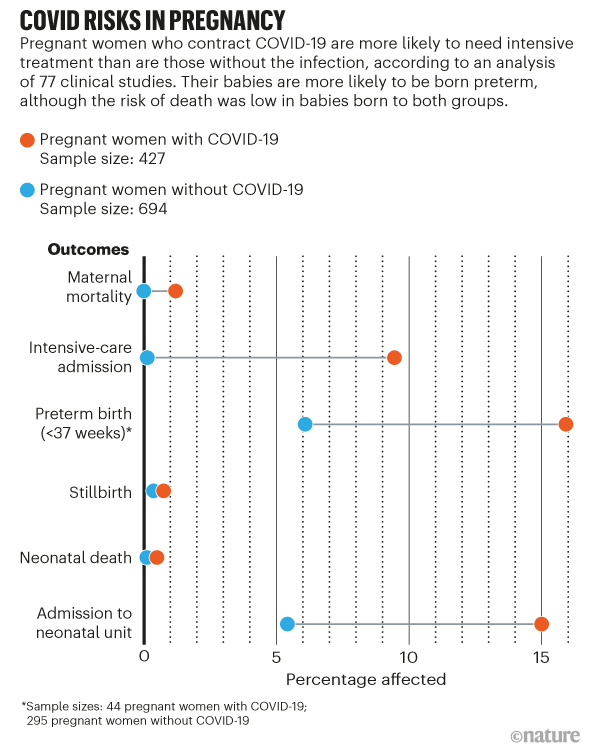
Developmental and reproductive toxicity studies show that the vaccines do not prevent female rodents becoming pregnant or harm the pups if. We report preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons from three US. However pregnant persons with Covid-19 are at increased risk for severe illness eg resulting in admission to an intensive care unit extracorporeal membrane oxygenation or mechanical ventilation and death as compared with nonpregnant persons of reproductive age.
Recent studies from Israel compared pregnant people who received an mRNA COVID-19 vaccine with those who did not. Vaccinated mothers and mothers with previous infection generated and transferred protective IgG antibodies across the placenta. In a UK study of pregnant women with COVID-19 disease serious enough to require hospital admission most infected in the second or third trimester only 6 of 265 babies tested positive for COVID.
A total of 131 reproductive-age vaccine recipients 84 pregnant 31 lactating and 16 nonpregnant women were. Moderna COVID-19 Vaccine mRNA-1273 Observational Pregnancy Outcome Study The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. The study provides the largest peer-reviewed evaluation of the safety of a COVID-19 vaccine in a nationwide mass-vaccination setting.
The eligibility period for a booster dose is based on several factors including. Listing a study does not mean it has been evaluated by the US. Scientists found that vaccination lowered the risk of infection from the virus that causes COVID-19.
As for the COVID-19 vaccines we currently have the researchers say that women should make sure to follow the recommended dosing schedule. Miscarriage typically occurs in about 11-16 of pregnancies and this study found miscarriage rates. A shocking new study published in the New England Journal of Medicine reveals that when pregnant women are given covid vaccinations during their first or second trimesters they suffer an 82 spontaneous abortion rate killing 4 out of 5 unborn babies.
Male fetuses linked to lower antibody production transfer In the second study the researchers found that pregnant women infected with COVID-19 and who were carrying male fetuses had lower COVID-specific IgG titers and lower COVID. Studies Confirm COVID-19 mRNA Vaccines Safe Effective for Pregnant Women. A clinical trial of Comirnaty is underway in the US and further real-world evidence is being gathered10 There are still very limited data on the safety of viral vector vaccines such as COVID-19 Vaccine AstraZeneca in pregnancy.
The expectation was that they would work just as well to protect pregnant women. 5 Furthermore pregnant persons with Covid-19 might be at increased risk for adverse pregnancy outcomes such as preterm birth as.
Pregnant Or Worried About Infertility Get Vaccinated Against Covid 19
Covid 19 Vaccine Studies Hse Ie

Coronavirus Disease 2019 Vaccine Response In Pregnant And Lactating Women A Cohort Study American Journal Of Obstetrics Gynecology

Pfizer Biontech To Trial Covid 19 Vaccine In Pregnant Women And Children
Covid 19 In Pregnancy Maternal Care Maternal Fetal Care High Risk Obstetrics Ur Medicine Obstetrics Gynecology University Of Rochester Medical Center
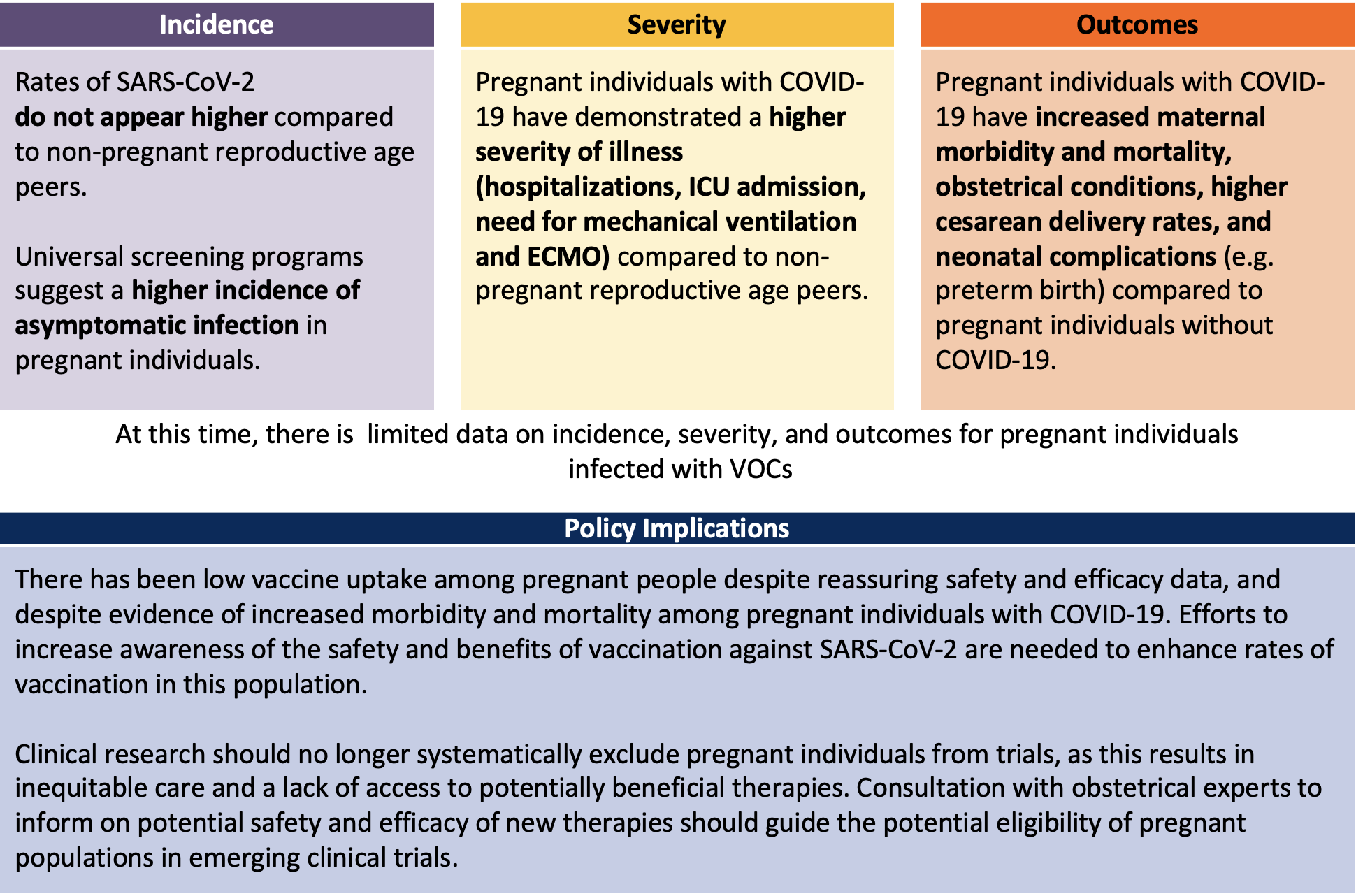
Frontiers Covid 19 Vaccination In Pregnancy And Lactation Current Research And Gaps In Understanding Cellular And Infection Microbiology

Coronavirus Disease 2019 Vaccine Response In Pregnant And Lactating Women A Cohort Study American Journal Of Obstetrics Gynecology

Covid 19 Ema Sets Up Infrastructure For Real World Monitoring Of Treatments And Vaccines European Medicines Agency
Covid 19 Vaccines And Pregnancy Johns Hopkins Bloomberg School Of Public Health

Clinical Manifestations Risk Factors And Maternal And Perinatal Outcomes Of Coronavirus Disease 2019 In Pregnancy Living Systematic Review And Meta Analysis The Bmj
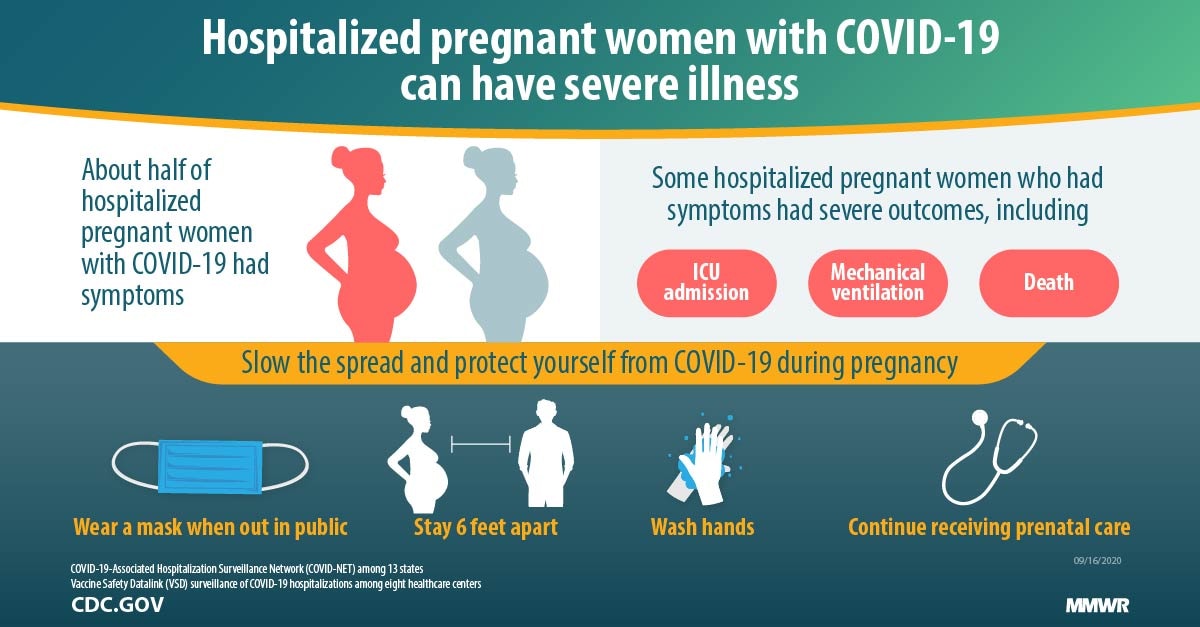
Characteristics And Maternal And Birth Outcomes Of Hospitalized Pregnant Women With Laboratory Confirmed Covid 19 Covid Net 13 States March 1 August 22 2020 Mmwr

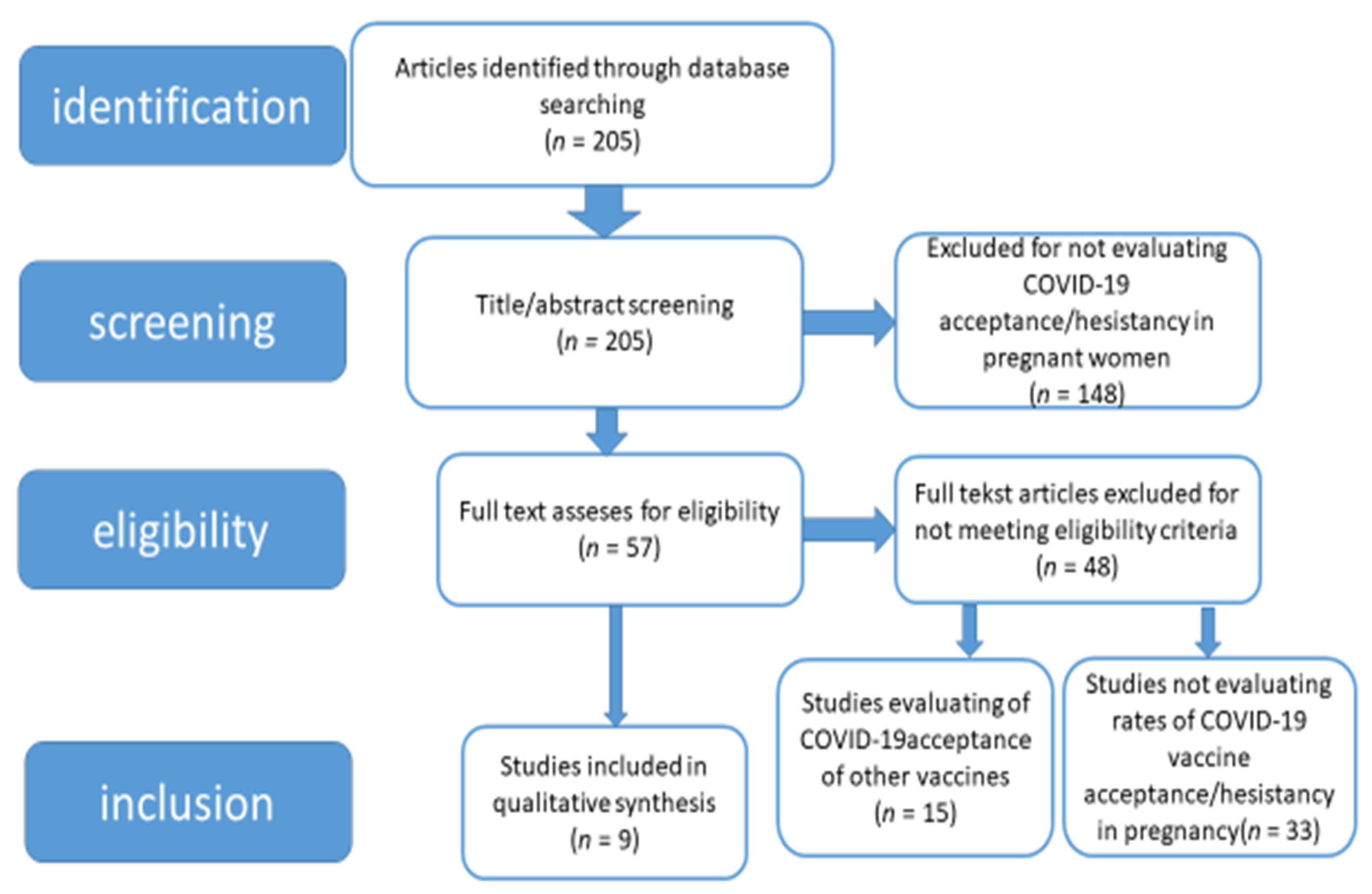
Medicina Free Full Text The Approach Of Pregnant Women To Vaccination Based On A Covid 19 Systematic Review Html
Covid 19 And Pregnancy In Massachusetts Mass Public Health Blog

Stiko Recommends Covid Vaccine For All Pregnant Women In Germany
Pregnant Women In Uk Given Green Light To Have Covid Jab Coronavirus The Guardian
Ohsu Studies Impacts Of Covid 19 Infection Vaccination On Immune System During Pregnancy Lactation Ohsu News