Covid Vaccine Pregnancy Trial / Delta Increases Covid 19 Risks For Pregnant Women Pfizer Biontech Vaccine Antibodies Gone By 7 Months For Many Reuters
NCT04816643 NCT04754594 NCT04955626 NCT05035212. Pregnant women are excluded from COVID-19 vaccines says a May 2020 report in the US National Library of Medicine.
Pfizer Study Of Covid 19 Vaccine In Pregnant Women Delayed By Slow Enrollment Wsj
It is notable that as of April 26 2021 more than 100000 pregnant women reported having received a Covid-19 vaccination and yet only a small fraction 47 have enrolled in the v.
Covid vaccine pregnancy trial. Although studies are beginning to focus on these vaccines and pregnancy it will be months or even years before we have results. The advisory committee for the Centers for Disease Control and Prevention is set to discuss the covid-19 paediatric vaccine trial. This episode dives into the available evidence on the safety and efficacy of COVID-19 vaccines during pregnancy so you can make an informed decision on whether to.
The Moderna COVID-19 Vaccine Pregnancy Registry will collect primary data from pregnant women who have received the Moderna COVID-19 vaccine and their healthcare providers HCPs. The placebo-controlled observer-blinded study will track the safety tolerability and. Among 621370 trials in the World Health Organization International Clinical Trials Registry 927 015 were COVID-19 related.
Antibodies made after a pregnant person received an mRNA COVID-19 vaccine were found in umbilical cord blood. Prior to conducting their COVID-19 vaccine clinical trial in pregnant women Pfizer and BioNTech completed a developmental and reproductive toxicity DART study with BNT162b2 which was required by the regulatory authorities before starting the study in pregnant women. Currently three COVID-19 vaccines.
Clinical trials have shown that COVID-19 vaccines are remarkably effective in protecting those age 12 and up against infection by the coronavirus SARS-CoV-2. Food and Drug Administration. Since Jackson reported problems with Ventavia to the FDA in September 2020 Pfizer has hired Ventavia as a research subcontractor on four other vaccine clinical trials covid-19 vaccine in children and young adults pregnant women and a booster dose as well an RSV vaccine trial.
Some COVID-19 vaccine makers have since planned to enroll pregnant people in their clinical trials. This means there is currently very limited clinical trial data on the immune response and side effects caused by the vaccines for these women. The initial advice from immunisation expert groups was therefore cautious and COVID-19 vaccines were not routinely recommended in pregnancy.
Participants have been given shots in a large-scale clinical trial to assess the safety and efficacy of their COVID-19. Vaccines are not provided to participants as part of the study protocol. COVID vaccine trial for pregnant women Pfizer and BioNTech say the first US.
While there is now evidence available to show that Covid-19 vaccines are safe for people who are pregnant or breastfeeding and their infants with more than 130000 people who received one while pregnant or breastfeeding joining the V-safe registry in the US the pandemic has shone a light on the historic failing and exclusion of pregnant people in clinical trials. Those studies showed no evidence of fertility or reproductive toxicity in animals. Clinical trials are now getting underway to address COVID-19 vaccine safety and efficacy in pregnant women.
Last week February 18 Pfizer and BioNTech announced a global Phase 23 trial of their COVID-19 vaccine in 4000 healthy pregnant women. Pregnant people were shut out of Covid vaccine trials with disastrous results. We have summarized several academic papers that investigate outcomes of COVID-19 infection and the vaccines against it among pregnant individuals to help journalists bolster their reporting with data.
The Joint Committee on Vaccination and Immunisation JCVI has advised that pregnant women should be offered COVID-19 vaccines at the same time as people of the same age or risk group. By Patty McMurrayPublished November 2 2021 100 Percent Fed Up After re-analyzing a study performed by Centers for Disease Control and Prevention CDC researchers a peer-reviewed study has called for the immediate withdrawal of mRNA COVID vaccines for pregnant women those breastfeeding those of childbearing age and children after their shocking study reveals stunning. In the USA.
The CDC looks to of manipulated study data to show the Covid-19 Vaccines are safe for Pregnant Women as researchers discover 91 of pregnancies resulted in miscarriage following Covid-19 Vaccination The Expose - h. Despite pregnancy increasing COVID-19 risks clinical trials did not include pregnant participants. Pregnancy has been excluded from past pandemic research say experts who study immunology history.
Scientists found that vaccination lowered the risk of infection from the virus that causes COVID-19. Go to Top of Page Study Description Study Design Groups and Cohorts Outcome Measures Eligibility Criteria Contacts and Locations More Information. The countrys largest clinical trial investigating the best gap between first and second COVID-19 vaccine doses for pregnant women is being launched in England today Tuesday 3 August.
Only about 34 percent of pregnant adults are fully vaccinated and more than 200 have died of the virus according. Vaccination of pregnant people builds antibodies that might protect their baby. The objective of the COVID-19 Vaccines International Pregnancy Exposure Registry C-VIPER is to evaluate obstetric neonatal and infant outcomes among women vaccinated during pregnancy to prevent COVID-19.
The expectation was that they would work just as well to protect pregnant women. In addition to vaccination protecting women against Covid-19 and its complications during pregnancy emerging evidence has shown transplacental transfer of severe acute respiratory syndrome. When pregnant people receive an mRNA COVID-19 vaccine during pregnancy their bodies build antibodies against COVID-19 similar to non-pregnant people.
Specifically the C-VIPER will estimate the risk of obstetric outcomes abortion antenatal bleeding dysfunctional labor gestational diabetes hypertensive disorders of pregnancy. Pregnant women are more likely to develop severe COVID-19 or die from the disease but are excluded from clinical trials with new vaccines. This means COVID-19 vaccination during pregnancy.
Pregnant people were not included in the first clinical trials for COVID-19 vaccines so at the time of initial guidance there was limited evidence confirming the safety of COVID-19 vaccines during pregnancy. Studies Confirm COVID-19 mRNA Vaccines Safe Effective for Pregnant Women. While pregnant and lactating women were largely not included in COVID-19 vaccine trials two studies yesterday in Science Translational Medicine look at how the mRNA COVID-19 vaccines affect the groups differently than nonpregnant women and at how the sex of the fetus may affect maternal immune response and vertical antibody transmission.
Their infants also will be enrolled in the study. Primary Outcome Measures. Investigators will enroll up to 750 pregnant individuals and 250 postpartum individuals within two months of delivery who have received or will receive any COVID-19 vaccine authorized or licensed by the US.
The Truth About Pregnancy Risks And Covid 19 Vaccines Los Angeles Times

Covid Vaccines Protect Pregnant Women Study Confirms The New York Times
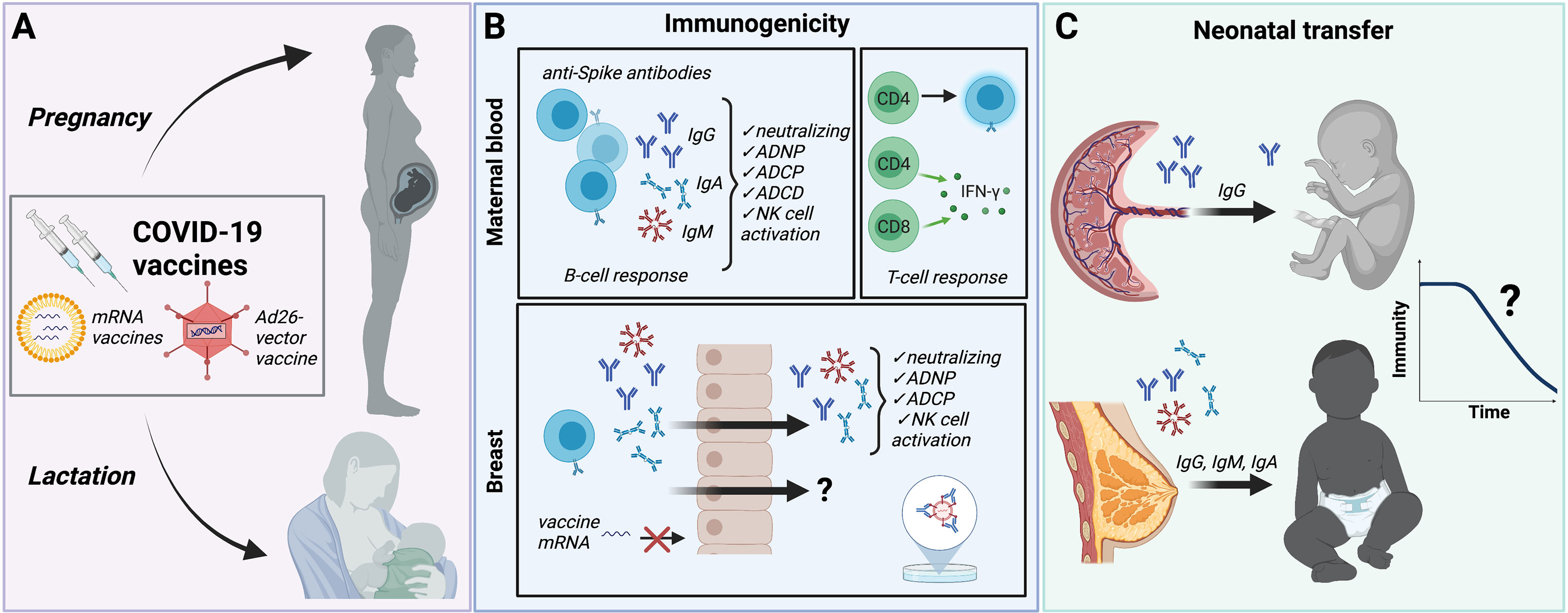
Frontiers Covid 19 Vaccination In Pregnancy And Lactation Current Research And Gaps In Understanding Cellular And Infection Microbiology

Covid Vaccine And Pregnancy What You Need To Know
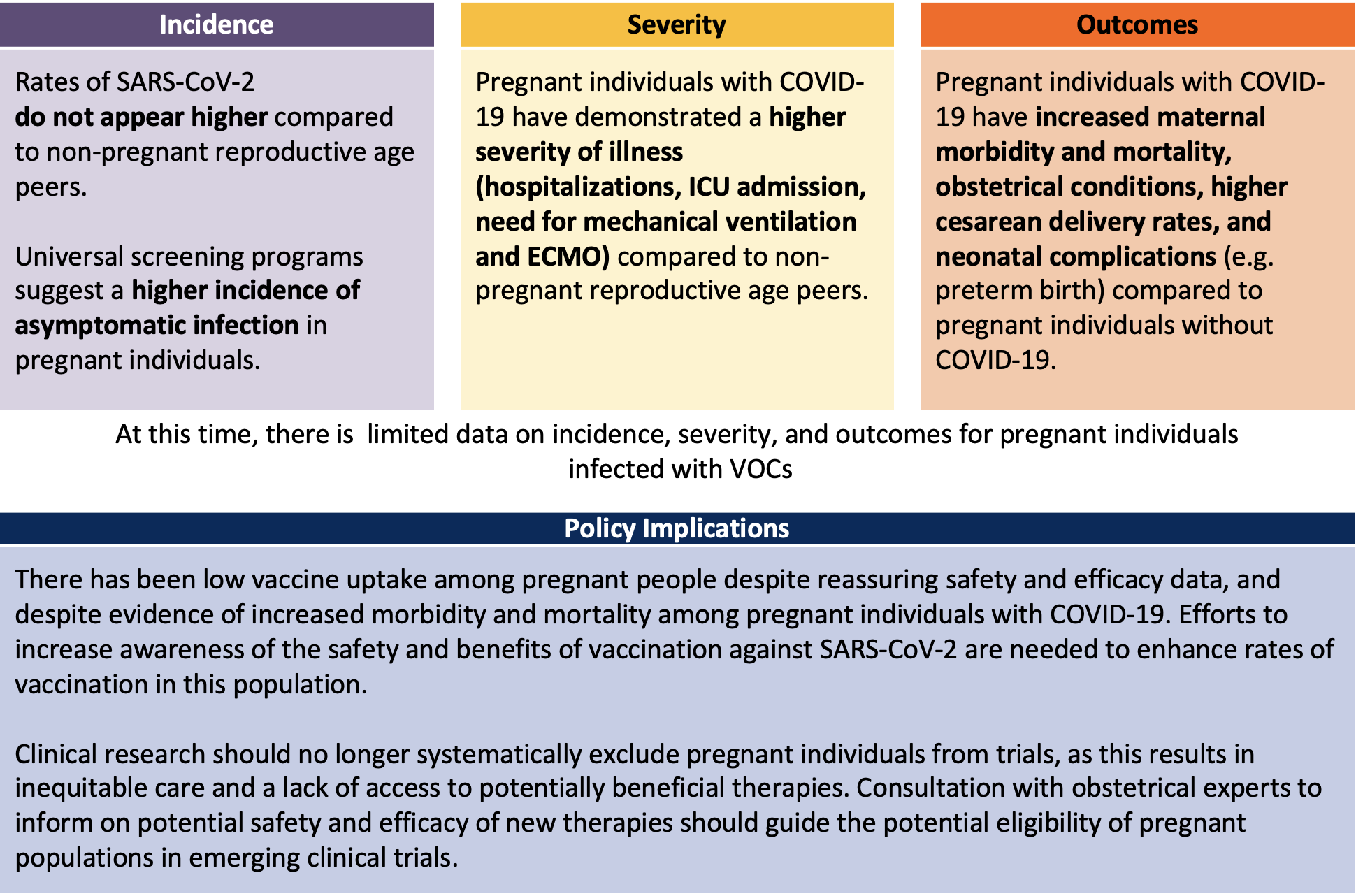
The Incidence Severity And Management Of Covid 19 In Critically Ill Pregnant Individuals Ontario Covid 19 Science Advisory Table

Jci Covid 19 Vaccine Testing In Pregnant Females Is Necessary

Delta Increases Covid 19 Risks For Pregnant Women Pfizer Biontech Vaccine Antibodies Gone By 7 Months For Many Reuters

I Got Vaccinated While Pregnant Please Study People Like Me Stat

Cdc Recommends Pregnant Women Get Covid Vaccine After Study Shows It S Safe

Should Pregnant Women Be Vaccinated Against Covid 19
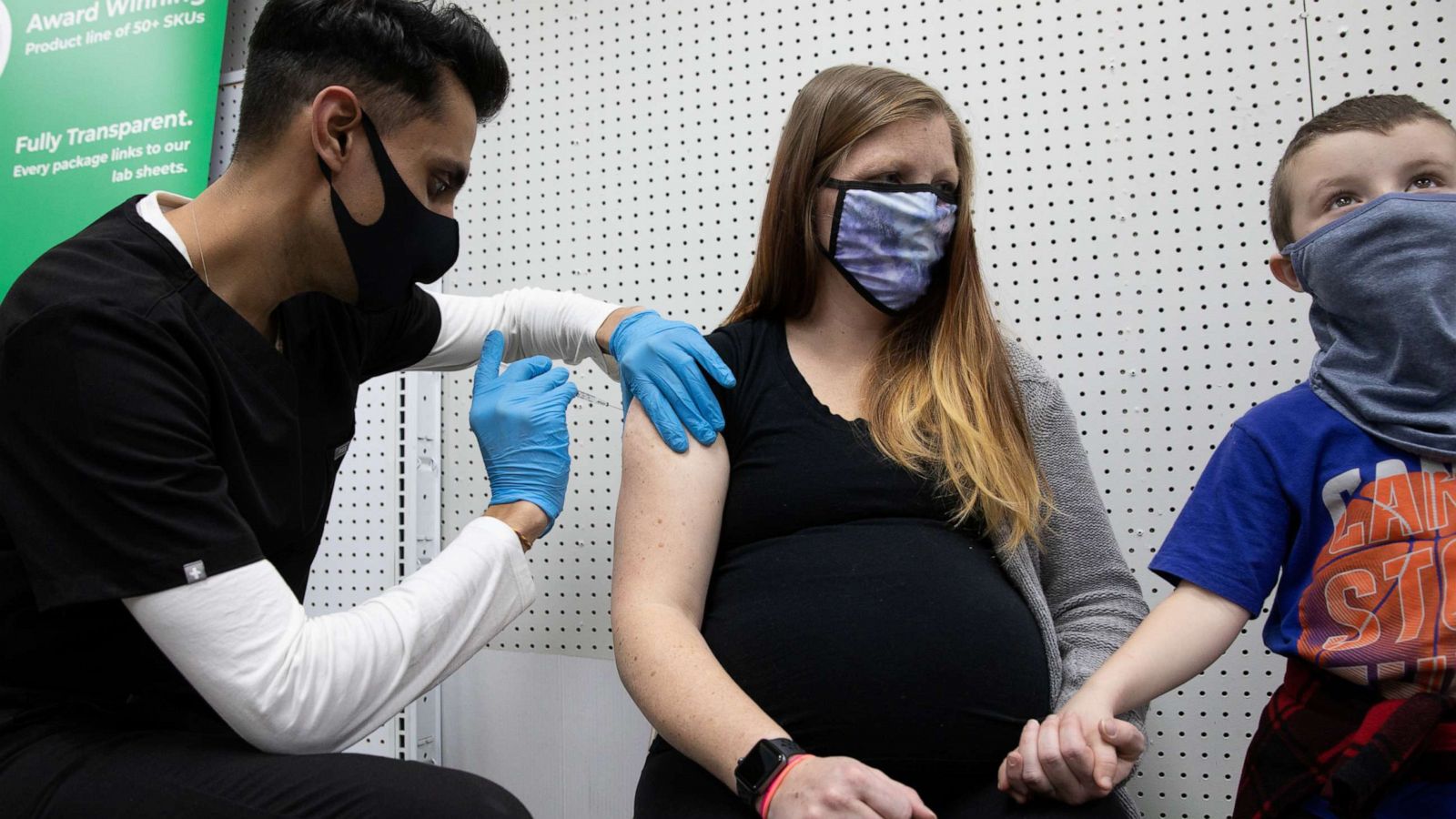
Pfizer And Moderna Are Safe And Effective In Pregnant Women Provide Antibodies To Newborns Abc News

Should You Get The Covid 19 Vaccine While Pregnant Here S What Experts Say
Nih Begins Clinical Trial Testing Covid 19 Vaccine In Pregnant Women Reuters

Lack Of Trials In Pregnant People Driving Covid 19 Vaccine Hesitancy
The Covid 19 Vaccine And Pregnancy Things To Know Hub
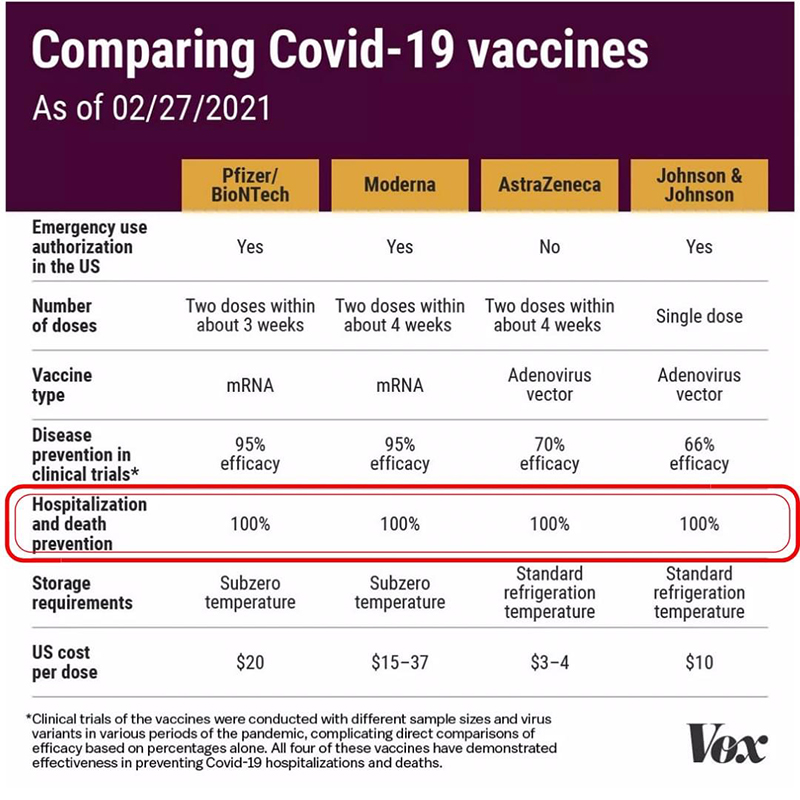
Covid 19 In Pregnancy Maternal Care Maternal Fetal Care High Risk Obstetrics Ur Medicine Obstetrics Gynecology University Of Rochester Medical Center

Coronavirus Disease 2019 Vaccine Response In Pregnant And Lactating Women A Cohort Study American Journal Of Obstetrics Gynecology
The Covid 19 Vaccine And Pregnancy What You Need To Know Johns Hopkins Medicine
No Placental Damage Observed In Case Study Of Pregnant Women With Mrna Covid 19 Vaccinations